- Home
- Industry
- Pharmaceutical & Vaccine Cold Chain Solutions

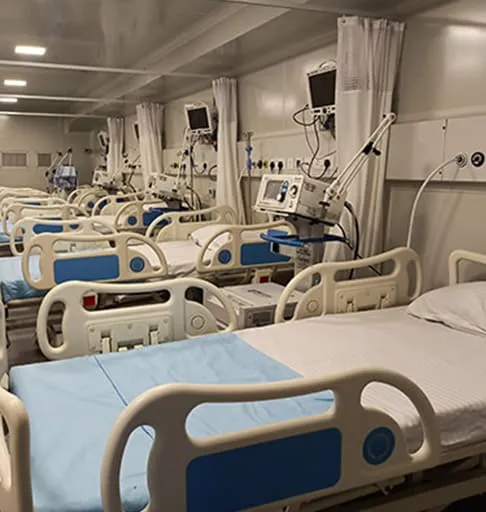
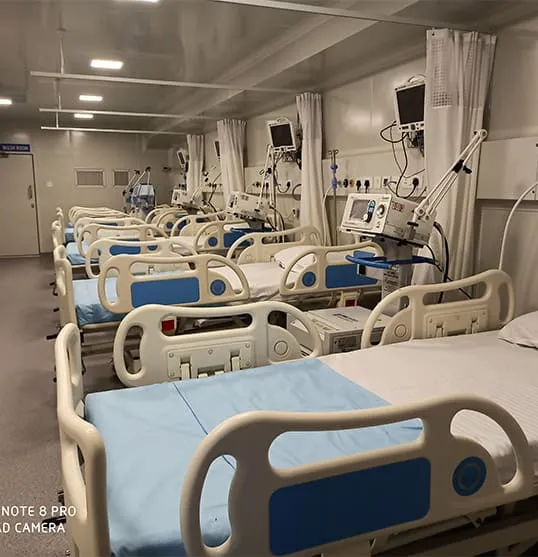
The pharmaceutical and vaccine industry requires precise temperature-controlled environments to maintain product efficacy and safety. Improper storage and transportation can lead to degradation, rendering medications and vaccines ineffective. Rinac India Limited provides state-of-the-art cold chain solutions designed to meet stringent regulatory requirements and ensure the integrity of pharmaceutical products from production to distribution.